 Spectroscopy measures the energy- or wavelength-dependence of how a material responds to light. It can be applied for light of any region of the electromagnetic spectrum, and will most often measure how that light is absorbed or reflected by materials. Light interacts with the fundamental charged particles – electrons and protons – in materials, and spectroscopic methods are used most often to measure the electronic properties of materials or the response of the electronic levels to light.
Spectroscopy measures the energy- or wavelength-dependence of how a material responds to light. It can be applied for light of any region of the electromagnetic spectrum, and will most often measure how that light is absorbed or reflected by materials. Light interacts with the fundamental charged particles – electrons and protons – in materials, and spectroscopic methods are used most often to measure the electronic properties of materials or the response of the electronic levels to light.
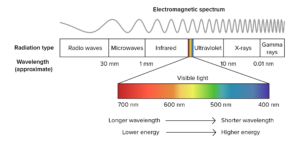 Geoscientists use spectroscopic methods over a wide range of the electromagnetic spectrum to measure different energy scales of minerals and geomaterials. Synchrotrons are especially suited for spectroscopies with X-rays, but are also valuable sources of intense and bright beams on other parts of the electromagnetic spectrum, notable the infrared region. SEES has several beamlines that support a variety of different kinds of spectroscopies that are used by geophysicists and geochemists.
Geoscientists use spectroscopic methods over a wide range of the electromagnetic spectrum to measure different energy scales of minerals and geomaterials. Synchrotrons are especially suited for spectroscopies with X-rays, but are also valuable sources of intense and bright beams on other parts of the electromagnetic spectrum, notable the infrared region. SEES has several beamlines that support a variety of different kinds of spectroscopies that are used by geophysicists and geochemists.
Infrared Spectroscopy
Infrared (IR) spectroscopy is used to study how molecules and solids absorb infrared radiation or heat. This energy range is associated with vibrations of atomic bonds, and IR spectroscopy is used to probe vibrational and rotational modes of molecules, typically looking at resonances in functional groups like O-H, C-O, C-H, C-C, N-H, N-O, N-N, and so forth. While conventional lab-based instruments are widely used for IR measurements, the intense beams from synchrotron sources make them excellent sources for these measurements. SEES supports IR synchrotron source at NSLS-II that is intense enough to allow measurements on samples at high pressure in diamond anvil cells. This allows geoscientists to use IR to measure things like how water and OH groups incorporated into hydrated minerals change with pressure.
Nuclear Resonance and Mössbauer Spectroscopy
Mössbauer spectroscopy is an important spectroscopic tool for geologists. This relies on an extremely sharp resonance in the absorption and emission of X-rays that happens when in certain isotopes.
The Mössbauer effect is especially important for geologists because one of the recoilless isotopes is 57Fe, and because the Mössbauer spectra is very good at distinguishing different chemical states for iron. Conventional Mössbauer measurements use a 57Co source which emits X-rays at the resonance during a beta-capture process (around 14.4 keV, and with an energy resolution around 1 μeV), and samples are often enriched in 57Fe.
Synchrotron Mössbauer measurements will select the high-resolution X-ray energy needed from the synchrotron radiation. Measurements can made either an “energy domain” or “time domain”, which then leads to variations in method under the broader term Nuclear Resonant Scattering. Geoscientists use SEES-supported beamline at APS to study Fe in minerals and glasses at extreme conditions using samples in diamond-anvil cells.
X-ray Fluorescence Spectroscopy
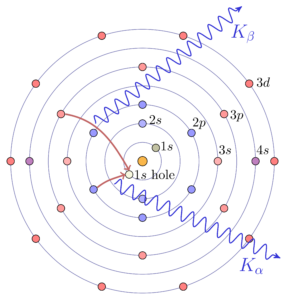 The core electrons of atoms are bound with energies in the same energy range (approximately 50 to 500,000 electron Volts) as X-rays. For each element, the set of electron energy binding energies for its core electrons is separated into distinct orbitals, each with precisely defined binding energies, (energy widths ~ 1 to 10 eV), and the energies of these levels increase smoothly with atomic number. If a deep core electron is removed from an atom, an electron from a higher-energy level will move into the lower energy level, and the difference in the energy levels will be released as light – a fluorescent X-ray – that has an energy characteristic for the element. X-ray Fluorescence (XRF) Spectroscopy uses these facts to identify the atomic elements in a material from the energies emitted by a sample that is excited by shining high-energy X-rays or electrons or other particles on it. Like XRD, XRF is widely used in many fields of science, either using lab-based electron microscopes or X-ray sources (even hand-held ones) to generate the incident energy and can be highly quantitatively precise at determining elemental abundances, typically to the parts-per-million level.
The core electrons of atoms are bound with energies in the same energy range (approximately 50 to 500,000 electron Volts) as X-rays. For each element, the set of electron energy binding energies for its core electrons is separated into distinct orbitals, each with precisely defined binding energies, (energy widths ~ 1 to 10 eV), and the energies of these levels increase smoothly with atomic number. If a deep core electron is removed from an atom, an electron from a higher-energy level will move into the lower energy level, and the difference in the energy levels will be released as light – a fluorescent X-ray – that has an energy characteristic for the element. X-ray Fluorescence (XRF) Spectroscopy uses these facts to identify the atomic elements in a material from the energies emitted by a sample that is excited by shining high-energy X-rays or electrons or other particles on it. Like XRD, XRF is widely used in many fields of science, either using lab-based electron microscopes or X-ray sources (even hand-held ones) to generate the incident energy and can be highly quantitatively precise at determining elemental abundances, typically to the parts-per-million level.
With a bright synchrotron source, XRF measurements can be made at extremes of beam sizes (down to 10 nanometers), sample pressure, and temperature, and high-quality spectra can be measured at 100 spectra per second or faster. This means that XRF can be used in a scanning probe (see XRF Mapping), which is very useful for Earth scientists studying complex mineral phases, aggregate rocks, soils, weathered systems, and biological or geo-biological samples, SEES supports several beamlines to do XRF and XRF Mapping, and several of these beamlines can also support other measurements such as XRD or XAS measurements.
X-ray Absorption Spectroscopy
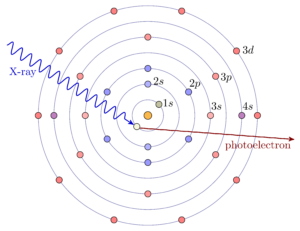 X-rays are absorbed by matter through the photo-electric effect, in which the energy of the X-ray is given to an electron in the material. As with X-ray Fluorescence, high energy X-rays have energies that are comparable to the deep core-level electrons of the elements, especially the heavier elements. When the X-ray energy increases through the binding energy of a particular core-level electron, it can be absorbed by that electron, giving a sharp increase in absorption. When the X-ray is absorbed, the energy is transferred to the core electron, and any excess energy goes to creating a photo-electron that can leave the atom. Chemical bonds between neighboring atoms are formed by exchanging the loosely-bound electrons with energies around the valence levels of each atom and so can alter the electronic state that is available to a photoelectron. This gives subtle changes in the energy threshold for X-ray absorption, and gives fine-structure to the measured X-ray absorption coefficient at and above the absorption edge.
X-rays are absorbed by matter through the photo-electric effect, in which the energy of the X-ray is given to an electron in the material. As with X-ray Fluorescence, high energy X-rays have energies that are comparable to the deep core-level electrons of the elements, especially the heavier elements. When the X-ray energy increases through the binding energy of a particular core-level electron, it can be absorbed by that electron, giving a sharp increase in absorption. When the X-ray is absorbed, the energy is transferred to the core electron, and any excess energy goes to creating a photo-electron that can leave the atom. Chemical bonds between neighboring atoms are formed by exchanging the loosely-bound electrons with energies around the valence levels of each atom and so can alter the electronic state that is available to a photoelectron. This gives subtle changes in the energy threshold for X-ray absorption, and gives fine-structure to the measured X-ray absorption coefficient at and above the absorption edge.
X-ray absorption Spectroscopy (XAS) measures the detailed energy dependence of this absorption around the binding energies of core-electrons to study the chemical state and detailed coordination environment around a selected element. As with XRF measurements, XAS can be measured on nearly any kind of sample, and at low concentrations. Because it requires scanning the incident X-ray energy across a particular absorption edge, it needs a broadly tunable X-ray source, which means that the technique is mostly done at synchrotron beamlines. XAS gives fairly direct and unique measurements of oxidation state and coordination chemistry for nearly any element. SEES supports several beamlines that combine XRF mapping and XAS measurements, which are used be Earth scientists to determine the oxidation state of elements and transition metals from S through Pu in complex mineral phases, aggregate rocks, volcanic magmas, meteorites, soils, weathered systems, and biological or geo-biological samples.
